How Unituxin® Works
Learn what Unituxin is and how it works in the body to help the immune system destroy neuroblastoma cells
What is
Unituxin?
What is
GD2?
How does
Unituxin work?
GD2 is also found on some healthy nerve cells, and the Unituxin antibody will bind to those cells as well. This causes pain. Pain is the most common side effect of Unituxin, and it will be managed proactively by the healthcare team. Learn more about pain and other side effects of treatment with Unituxin
Watch the video below to learn more about how Unituxin works
- Contents of the video about how Unituxin works
- 00:00 – Understanding neuroblastoma
- 01:29 – What is Unituxin?
- 03:21 – Brief overview of the immune system
- 04:51 – How does Unituxin work?
- 07:11 – Most common side effects
- 08:14 – Required pretreatment and guidelines for side effect management
- 13:14 – Indication, Boxed WARNING, and Important Safety Information
Understanding Neuroblastoma
Neuroblastoma is a cancer that arises from immature nerve cells. It is the most common cancer diagnosed in the first year of life, and the third most common childhood cancer overall. It can be hard to diagnose, partly because the symptoms can mimic other childhood illnesses. These symptoms can include fever, diarrhea, bone pain, abdominal pain, fatigue, shortness of breath, or dark circles around the eyes. When a child is diagnosed with neuroblastoma, the disease is categorized into risk groups (low, intermediate, or high-risk). There are many factors that doctors use to determine which risk group the disease belongs to, including the stage of disease, the age of the child, examination of the tumor under a microscope, and tests to identify genetic alterations within the tumor.
Treatment for neuroblastoma varies depending on the risk group of the disease. High-risk neuroblastoma treatment is different from treatment for low- and intermediate-risk neuroblastoma. The remainder of this presentation focuses on high-risk neuroblastoma. Typical treatment for high-risk neuroblastoma includes multiple courses of chemotherapy, surgery to remove the primary tumor, autologous stem cell transplant (using the patient's own stem cells), radiation to the tumor site, and then antibody therapy.
What is Unituxin?
Unituxin is a man-made antibody called a “monoclonal antibody” developed by scientists in a lab. Unituxin is used to treat children with high-risk neuroblastoma who have had some success with prior first-line treatments. Granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin- 2 (IL-2), and isotretinoin (also known as retinoic acid, RA) are part of the treatment regimen with Unituxin.
Unituxin has a Boxed WARNING, which includes Serious Infusion Reactions and Neurotoxicity. Unituxin is contraindicated in patients with a history of anaphylaxis to Unituxin (dinutuximab). Please see Important Safety Information at the end of this presentation and Full Prescribing Information, including Boxed WARNING, provided. For more information, visit Unituxin.com.
The pivotal trial for Unituxin was conducted by the Children’s Oncology Group. The study evaluated 226 children with high-risk neuroblastoma who had received prior therapy with some success. Half of the children were given Unituxin plus GM-CSF, IL-2, and isotretinoin. The other half of the children in the clinical study received isotretinoin alone. This study showed that after 2 years, event-free survival (which is survival without relapse, progressive disease, secondary cancer, or death) improved by 20% for children receiving the Unituxin antibody regimen (Unituxin, GM-CSF, IL-2, and isotretinoin), when compared to children that received isotretinoin alone.
Please see Important Safety Information at the end of this presentation and Full Prescribing Information, including Boxed WARNING, provided. For more information visit Unituxin.com.
To understand how Unituxin works, it’s important to first understand a little bit about how the immune system works. The immune system is made of an army of many different cells, including white blood cells, that work together to protect the body from getting sick. In a healthy body, white blood cells work together with other cells of the immune system to attack and destroy threatening or harmful cells. Threats can come in many forms, like bacteria or viruses. Antibodies are part of the immune system and help defend the body against threatening cells. They are y-shaped proteins that act like detectives looking for threatening or abnormal cells in the body. Antibodies recognize threatening cells by attaching to molecules on their surface. These surface molecules are called “antigens.” Once an antibody binds to an antigen on the threatening cell, it activates the immune system and enables white blood cells to find and destroy the threatening or abnormal cell.
Sometimes the body’s own cells can become a threat. An example of this is cancer, where because of either genetic or environmental causes, normal cells do not develop properly or can change to become harmful. Sometimes cancer cells live longer than healthy cells and can multiply quickly. In a child with neuroblastoma, the immune system is weak (also called immunosuppressed), and neuroblastoma cells are able to hide from the immune system. If the immune system cannot find the cancer cells, then it cannot make the antibodies it needs to properly defend itself.
How does Unituxin work?
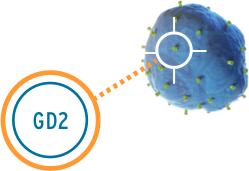
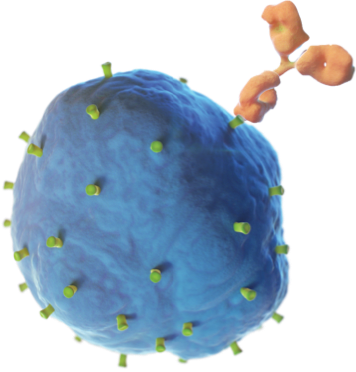
Unituxin targets an antigen found on neuroblastoma cells called the GD2 antigen. GD2 is also found on some non-cancerous cells in the body like nerve cells and pain fibers. The Unituxin antibody helps the body find the neuroblastoma cells by attaching to the GD2 antigen found on their cell surface. Once attached, the antibody sends a signal to the immune system, and white blood cells are sent to destroy the neuroblastoma cells. Because GD2 is found on some healthy nerve cells, the Unituxin antibody will bind to those cells as well. This can result in pain and peripheral neuropathy. Pain is the most common side effect of Unituxin. All patients receive pain medications before, during, and after the Unituxin infusion.
The following warnings and precautions reference some of the serious adverse reactions reported with Unituxin. They include: serious infusion reactions, neurotoxicity, neurological disorders of the eye, prolonged urinary retention, transverse myelitis, reversible posterior leukoencephalopathy syndrome, capillary leak syndrome, low blood pressure, systemic infection, bone marrow suppression, electrolyte abnormalities, and atypical hemolytic uremic syndrome. Tell your physician if you’re pregnant before taking Unituxin. Unituxin may cause harm to an unborn child. Please see Important Safety Information at the end of this presentation and Full Prescribing Information, including Boxed WARNING, provided. For more information, visit Unituxin.com.
Other medications in the Unituxin antibody regimen
Granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and isotretinoin are part of the treatment regimen with Unituxin. GM-CSF and IL-2 are cytokines. Cytokines help the immune system by boosting the number of white blood cells. Isotretinoin, also called retinoic acid (RA), has been shown to encourage some neuroblastoma cells to mature.
Side effects
The following is a list of the most common side effects seen in 25% or more of patients receiving Unituxin.
- Pain and peripheral neuropathy
- Fever
- Slow blood clotting
- Reduced ability to fight infections (low levels of white blood cells of various kinds)
- Infusion reactions
- Low blood pressure
- Low levels of sodium in the blood
- Increased levels of the enzyme alanine aminotransferase in the blood
- Low red blood cell count
- Vomiting
- Diarrhea
- Low levels of potassium in the blood
- Capillary leak syndrome
- Hives
- Low levels of albumin in the blood
- Increased levels of the enzyme aspartate aminotransferase
- Low levels of calcium in the blood
Tell your healthcare professional about any side effect seen during treatment with Unituxin. These are not all the possible side effects of Unituxin.
There are many expected side effects with Unituxin antibody therapy. Some of the most common side effects are pain and peripheral neuropathy, infusion reactions, capillary leak syndrome, and hypotension. For a complete list of side effects seen with Unituxin, please see Full Prescribing Information, including Boxed WARNING, provided. For more information, visit Unituxin.com.
Your healthcare team will use required pretreatment medications to help lessen some of these side effects. They include an intravenous infusion of normal saline; medications for allergic reaction, such as Benadryl; medications for fever, such as Tylenol; and strong medications for pain, such as morphine. Specific medicines used will be chosen by your healthcare provider. Some side effects of Unituxin may require your child’s infusion to be slowed, paused, or even stopped until the side effects have resolved. Some side effects may require permanent discontinuation of Unituxin. Let’s take a closer look at some of the more common side effects of Unituxin.
Pain may feel dull, sharp, or tingling like ‘pins and needles.’ During the study, 85% of patients had pain during the Unituxin infusion and 2 to 9% experienced severe (Grade 3) peripheral sensory neuropathy. To help lessen the pain, your child will receive a dose of strong pain medication (such as morphine) prior to the Unituxin infusion and then will receive a continuous infusion of pain medication during the Unituxin infusion. Your child’s pain will be monitored closely while the Unituxin is infusing. Your child may require additional doses of pain medication or an increase in the dose of the pain medication. Specific medicines used will be chosen by your healthcare provider.
Fevers and Infusion Reactions
Fevers and infusion reactions are both defense responses by the body and are a sign that the immune system has been activated. 72% of patients experienced fever during the Unituxin infusion. 60% of patients had symptoms of an infusion reaction. Your child will be given Tylenol to control fever and medicine (such as Benadryl) during the infusion to help prevent any reactions. Your child’s temperature will be checked frequently during the infusion. Your healthcare providers will follow their hospital guidelines for fever (which may include blood cultures and/or antibiotics). Tell your healthcare providers right away if your child has a rash, is coughing, if their lips or face is swelling, if they are having a hard time breathing, or if you are worried about any other symptoms. The infusion will be slowed down, stopped, or permanently discontinued, depending on severity, if your child develops a serious infusion reaction.
Capillary leak syndrome is when blood vessels are dilated and leak fluid into surrounding tissues. This can happen when the immune system is activated and there are high levels of cytokines. 40% of patients who received Unituxin developed capillary leak syndrome. To assess for signs of capillary leak, your child’s vital signs, weight, fluid intake, and urine output will be monitored closely. Make sure to always tell the nurse how much your child drinks and how much urine they produce. Tell your healthcare providers if you notice your child has any swelling, or if they feel lightheaded, dizzy, or weak. The infusion may be slowed down or stopped if your child develops moderate-to-severe capillary leak.
Low blood pressure
Low blood pressure, also known as hypotension, is when pressure within the blood vessels is less than normal. 60% of patients receiving Unituxin experienced low blood pressure. Low blood pressure can be a direct side effect of the Unituxin infusion. It can also be a late sign of capillary leak syndrome. Symptoms of low blood pressure may include dizziness, lightheadedness, nausea, or fainting. Your child will be given an intravenous infusion of normal saline, over one hour, prior to the start of Unituxin to help prevent low blood pressure. Your child’s blood pressure will be checked frequently during the Unituxin infusion. Tell your healthcare providers if your child is dizzy or lightheaded. The Unituxin infusion may be stopped if your child develops low blood pressure.
Side effects may still occur even with the use of required premedication. Slowing the infusion rate may help some side effects. Unituxin can be given over 10 to 20 hours. It is not uncommon to need to slow, pause, or even stop the Unituxin infusion until side effects resolve. The infusion will be slowed down, stopped, or permanently discontinued if your child develops a serious reaction. Please see full Unituxin Prescribing Information, including Boxed WARNING, for additional information on side effects and management of side effects.