Unituxin® MOA
How Unituxin works: targeting the GD2 antigen
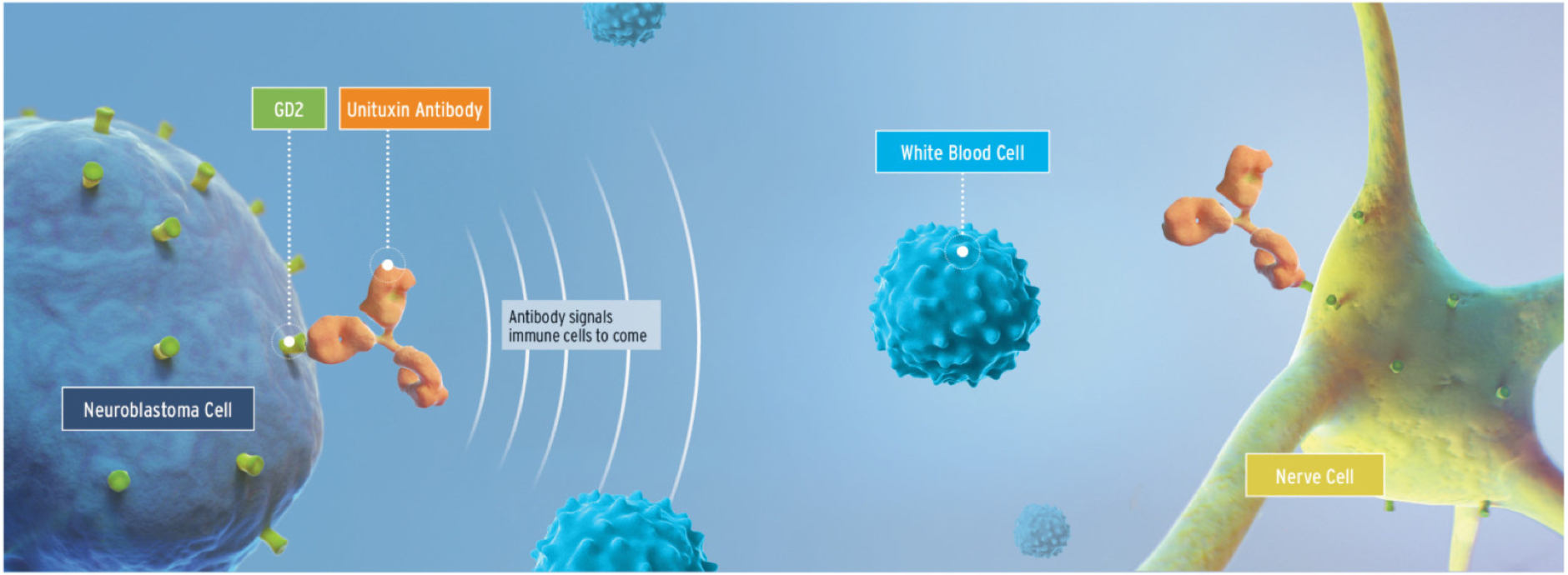
Unituxin is an anti-GD2 monoclonal antibody that induces tumor cell lysis via multiple mechanisms1
The glycolipid GD2 is an antigen that is expressed on both cancerous and noncancerous cells. GD2 is uniformly expressed by neuroblastoma cells in high amounts. In normal cells, its expression is limited to cells of neuroectodermal origin, including the central nervous system and peripheral nerves.1,2
Mechanism of Action
When Unituxin binds to the GD2 antigen on the surface of tumor cells, it induces cell lysis through antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity.1
Because GD2 is found on some healthy nerve cells, Unituxin binds to those cells, causing pain.3
Watch the video below to learn more about how Unituxin works
- Contents of this video
- 00:05 - What is Unituxin?
- 01:56 - How does Unituxin work?
- 02:40 - Most common adverse reactions
- 03:22 - Common side effects and required pretreatment and guidelines for side effect management
- 11:12 - Indication, Boxed WARNING, and Important Safety Information
What is Unituxin (dinutuximab)?
Unituxin is a chimeric, GD-2 binding monoclonal antibody indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and 13-cis retinoic acid (RA) for the treatment of pediatric patients with high-risk neuroblastoma who achieve at least a partial response to prior first-line multiagent, multimodality therapy.
Unituxin has a Boxed WARNING, which includes Serious Infusion Reactions and Neurotoxicity. Unituxin is contraindicated in patients with a history of anaphylaxis to Unituxin (dinutuximab). Please see Important Safety Information at the end of this presentation and Full Prescribing Information, including Boxed WARNING provided. For more information, visit Unituxin.com.
GD2 is a disialoganglioside antigen expressed on neuroblastoma cells and on other cells of neuroectodermal origin, including the central nervous system and peripheral nerves. Targeting GD2 on tumor cells using Unituxin has become standard of care in the treatment of high-risk neuroblastoma. In a healthy person, leukocytes of the innate immune system identify and eliminate pathogens. However, in a patient with neuroblastoma, multiple mechanisms allow the neuroblastoma cells to escape immune detection and may generate a directly immunosuppressive environment. In this kind of environment, the neuroblastoma tumor is able to grow. Early studies showed that anti-GD2 monoclonal antibodies prevented neuroblastoma cells from circumventing immune defenses.
How does Unituxin work?
The Unituxin antibody helps the body’s immune system detect neuroblastoma cells by targeting the GD2 antigen receptors and binding to them. Once bound to GD2, Unituxin triggers an immune response and induces cell lysis of neuroblastoma cells through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). It is important to understand that Unituxin also targets non-cancerous cells in the body that express GD2, specifically neurons, skin melanocytes, and peripheral pain fibers. Because the GD2 antigen is expressed on some nerve cells, the binding of Unituxin can cause pain and peripheral neuropathy in patients receiving this medication. In the process of ADCC, immune cells such as natural killer (NK) cells and granulocytes bind to the Unituxin antibodies. This triggers the release of cytotoxins leading to lysis of the neuroblastoma cells. Additionally, during the process of CDC, the c1 complement protein also adheres to the tumor-bound Unituxin antibodies, triggering the complement cascade. This results in the formation of the membrane attack complex, and ultimately leads to lysis of the tumor cells.
Based on their ability to stimulate the body's immune system in different ways, cytokines GM-CSF and IL-2 were included as part of the treatment regimen with Unituxin. GM-CSF is given with Unituxin in cycles 1,3 and 5 while IL-2 is given with Unituxin in cycles 2 and 4. GM-CSF has been shown both in vitro and in vivo to activate monocytes, macrophages, and dendritic cells, while IL-2 has been shown to activate NK cells. RA has been shown to induce differentiation of some neuroblastoma cells and is included in all 6 cycles of the antibody regimen.
The following is a list of warnings and precautions related to the most common serious adverse reactions reported with Unituxin.
Warnings and Precautions: Highlights from the Prescribing Information
- Neurological Disorders of the Eye: Interrupt Unituxin for dilated pupil with sluggish light reflex or other visual disturbances and permanently discontinue Unituxin for recurrent eye disorders or loss of vision
- Prolonged Urinary Retention and Transverse Myelitis: Permanently discontinue Unituxin and institute supportive care
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS): Permanently discontinue Unituxin and institute supportive care for signs and symptoms of RPLS
- Capillary Leak Syndrome and Hypotension: Administer required prehydration and monitor patients closely during treatment. Depending upon severity, manage by interruption, infusion rate reduction, or permanent discontinuation
- Infection: Interrupt until resolution of infection
- Bone Marrow Suppression: Monitor peripheral blood counts during Unituxin therapy
- Electrolyte Abnormalities: Monitor serum electrolytes closely
- Atypical Hemolytic Uremic Syndrome: Permanently discontinue Unituxin and institute supportive management
- Embryo-Fetal toxicity: May cause fetal harm. Advise females of reproductive potential of potential risk to a fetus and to use effective contraception
These highlights do not include all the information needed to use UNITUXIN safely and effectively. Please see Important Safety Information at the end of this presentation and Full Prescribing Information, including Boxed WARNING, provided. For more information, visit Unituxin.com.
It is common for patients to experience side effects, some of which are serious, during Unituxin antibody treatment. Some of these side effects can be directly related to the mechanism of action of the Unituxin while some are related to the immune response. Given these expected side effects, required pretreatment and guidelines for pain management are included in the prescribing information for Unituxin. Unituxin antibodies bind to GD2 on the surface of peripheral nerve fibers, which can result in mild to severe pain commonly experienced during and sometimes following the Unituxin infusion. Unituxin can also cause peripheral neuropathy. Severe neuropathic pain occurs in the majority of patients. Severe (Grade 3) peripheral sensory neuropathy ranged from 2% to 9% in patients with neuroblastoma. Therefore, aggressive pain management is essential when administering Unituxin and a continuous opioid infusion is required. Immediately prior to initiating the Unituxin infusion, a morphine bolus dose is given, followed by a continuous morphine infusion during the administration of Unituxin. Pain may be difficult to control and additional bolus doses, as well as titration of the continuous opioid medication, will likely be necessary. Consider using fentanyl or hydromorphone if morphine sulfate is not tolerated. If pain is inadequately managed with opioids, consider using gabapentin or IV lidocaine in conjunction with IV morphine.
Fevers and infusion reactions are common during Unituxin antibody therapy and are likely due to immune stimulation and cytokine release. To help prevent infusion reactions and fevers, patients are given antihistamines and acetaminophen 20 minutes prior to Unituxin infusion. Antihistamines are continued throughout the infusion every 4 to 6 hours as tolerated. Acetaminophen is continued throughout the infusion every 4 to 6 hours as needed. In patients with persistent fevers or pain, consider using Ibuprofen every 6 hours as needed. Immediately interrupt Unituxin for severe infusion reactions and permanently discontinue Unituxin for anaphylaxis.
Immune stimulation and cytokine release may also cause endothelial damage in capillaries which leads to increased vascular permeability and vasodilation, resulting in capillary leak syndrome. Capillary leak syndrome is characterized by intravascular fluid leakage into the interstitial space, which can lead to generalized edema and hypotension. To help maintain hydration and prevent hypotension a 0.9% sodium chloride infusion is administered over one hour prior to initiation of the Unituxin infusion. It is important to assess patients for signs of capillary leak syndrome by monitoring patient weight, vital signs, labs (hemoglobin, albumin, etc), and strict intake and output.
Side effects may still occur even with the use of required premedication. The rate of the Unituxin infusion can be modified to help mitigate side effects. Unituxin can be given over 10 to 20 hours. It is not uncommon to need to slow, pause or even stop the Unituxin infusion until side effects resolve. Please see full Unituxin Prescribing Information for recommendations on how to adjust the rate for specific side effects.
These tables show selected adverse reactions that occurred in at least 10% of patients, and they compare all grades and grades 3-4 AEs in the Unituxin antibody plus RA arm with those in the RA alone arm.
- Pain
- Pyrexia
- Edema
- Thrombocytopenia
- Lymphopenia
- Anemia
- Neutropenia
- Infusion reactions
- Hypotension
- Capillary leak syndrome
- Hemorrhage
- Hypertension
- Hyponatremia
- Hypokalemia
- Hypoalbuminemia
- Hypocalcemia
- Hypophosphatemia
- Hyperglycemia
- Hypomagnesemia
- Hypertriglyceridemia
- Decreased appetite
- Increased alanine aminotransferase
- Increased aspartate aminotransferase
- Increased serum creatinine
- Increased weight
- Vomiting
- Diarrhea
- Nausea
- Urticaria
- Hypoxia
- Tachycardia
- Sepsis
- Device-related infection
- Proteinuria
- Peripheral neuropathy
This table summarizes the adverse reactions that require permanent discontinuation of Unituxin.
Please see Full Prescribing Information, including Boxed WARNING, for Unituxin, provided. For more information, visit Unituxin.com.
References: 1. Unituxin [package insert]. Research Triangle Park, NC: United Therapeutics Corporation; 2020. 2. Yu AL, Gilman AL, Ozkaynak MF, et al; the Children's Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324-1334. 3. Bartholomew J, Washington T, Bergeron S, Nielson D, Saggio J, Quirk L. Dinutuximab: a novel immunotherapy in the treatment of pediatric patients with high-risk neuroblastoma. J Pediatr Oncol Nurs. 2017;34(1):5-12. doi:10.1177/1043454216659448.