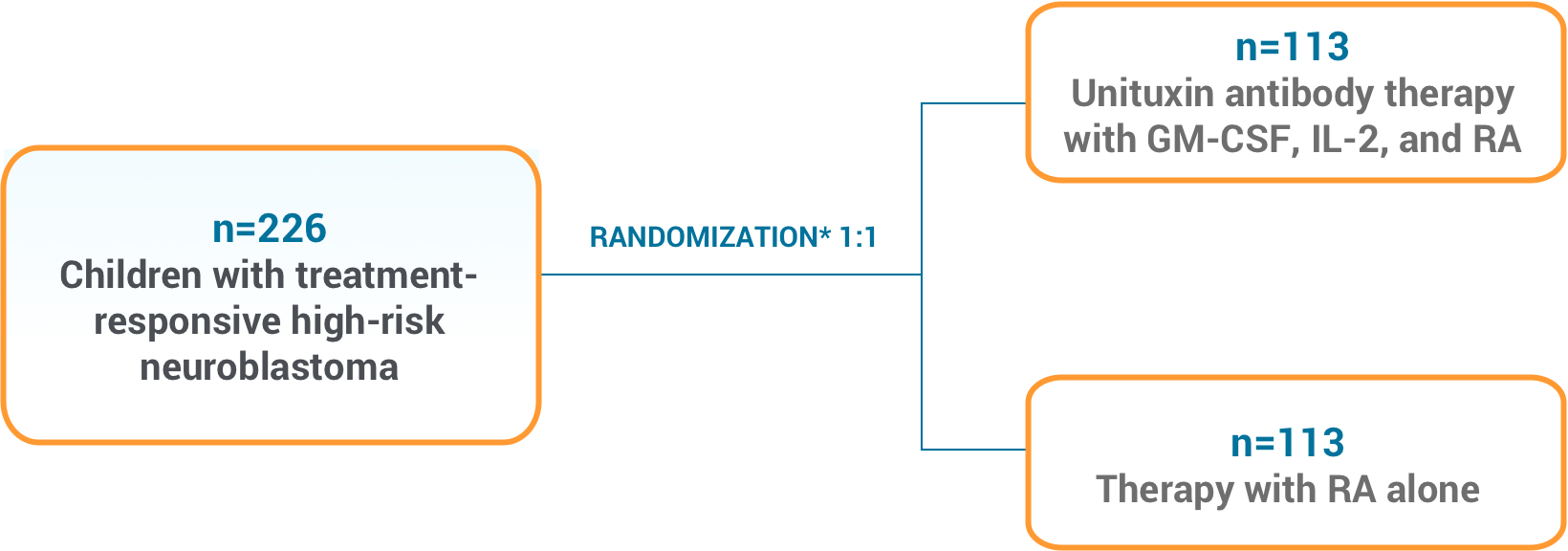
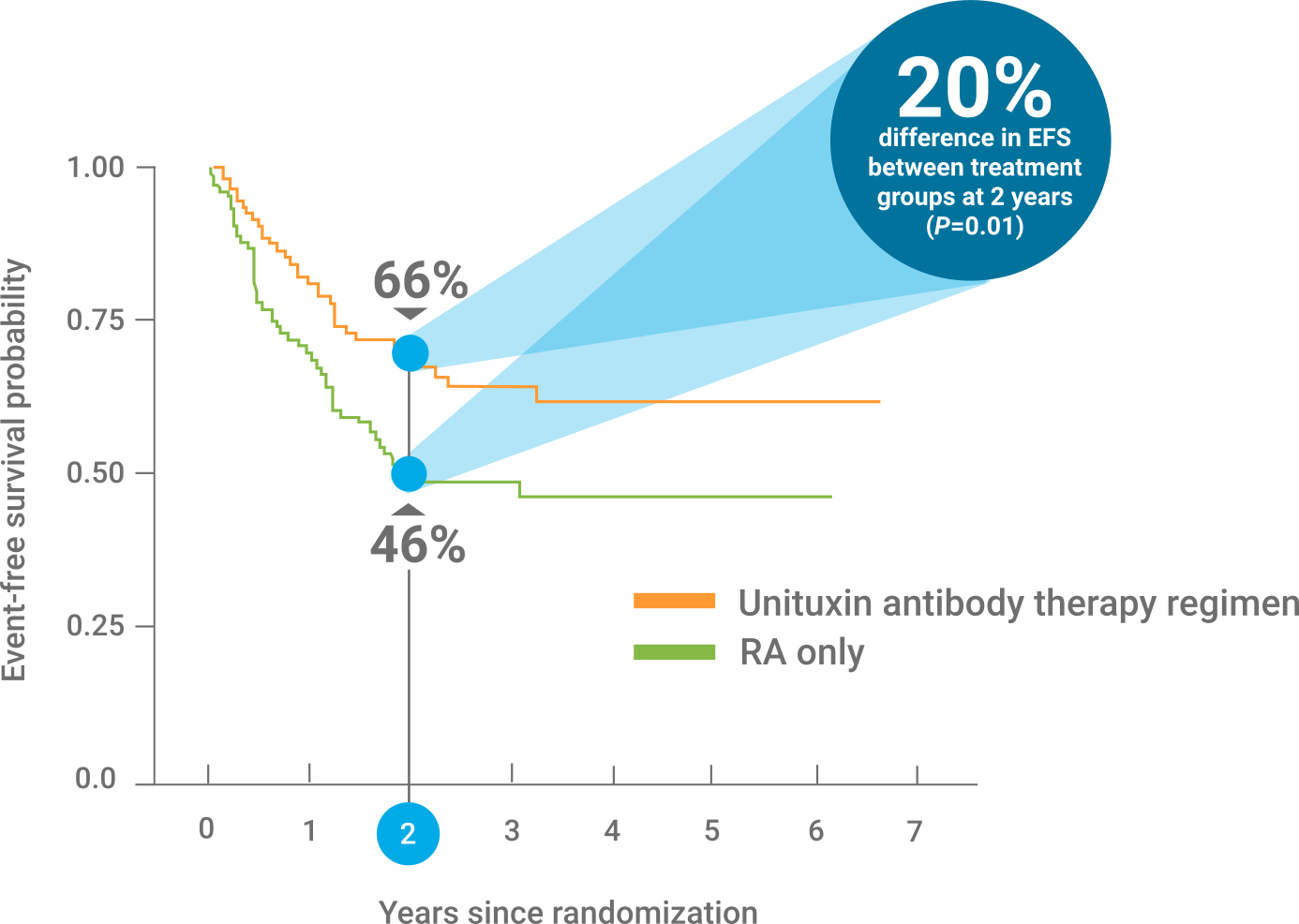
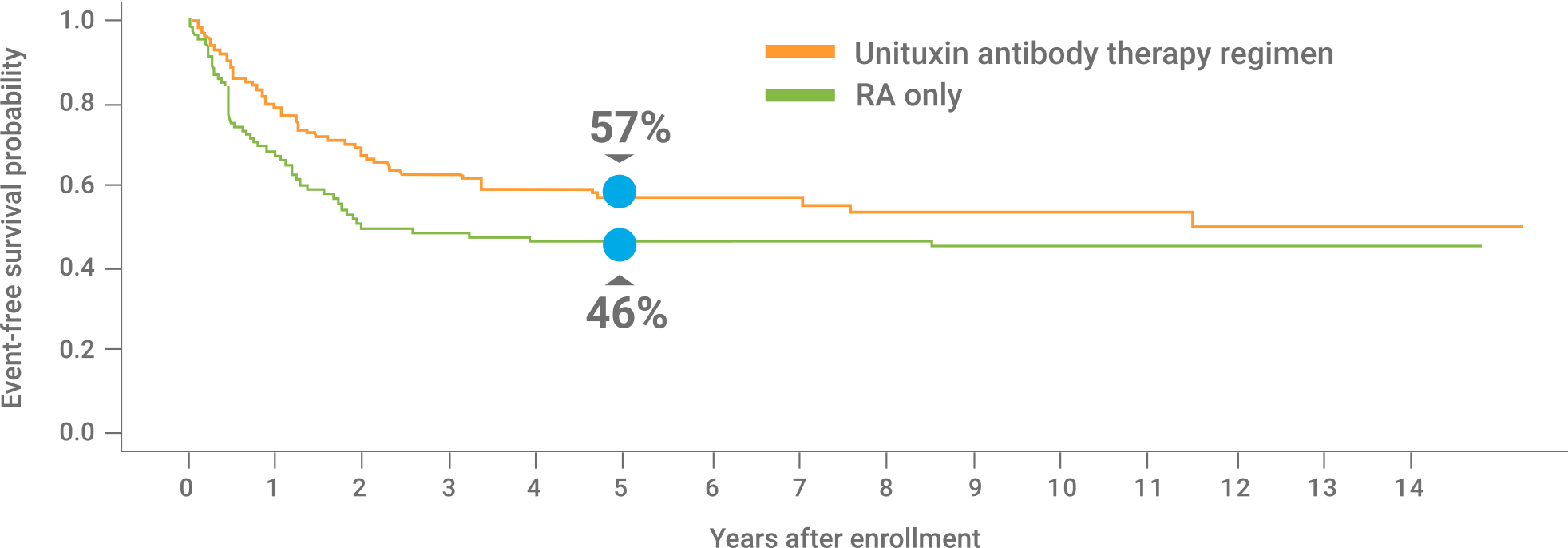
CONTRAINDICATIONS
Unituxin is contraindicated in patients with a history of anaphylaxis to dinutuximab.
WARNINGS AND PRECAUTIONS
Serious Infusion Reactions
-
Serious infusion reactions requiring urgent intervention including blood pressure support,
bronchodilator therapy, corticosteroids, infusion rate reduction, infusion interruption, or
permanent discontinuation of Unituxin included facial and upper airway edema, dyspnea,
bronchospasm, stridor, urticaria, and hypotension. Infusion reactions generally occurred during
or within 24 hours of completing the Unituxin infusion. Due to overlapping signs and symptoms,
it was not possible to distinguish between infusion reactions and hypersensitivity reactions in
some cases.
-
Severe (Grade 3 or 4) infusion reactions occurred in 35 (26%) patients in the
Unituxin/13-cis-retinoic acid (RA) group compared to 1 (1%) patient receiving RA alone. Severe
urticaria occurred in 17 (13%) patients in the Unituxin/RA group but did not occur in the RA
group. Serious adverse reactions consistent with anaphylaxis and resulting in permanent
discontinuation of Unituxin occurred in 2 (1%) patients in the Unituxin/RA group. Additionally,
1 (0.1%) patient had multiple cardiac arrests and died within 24 hours after having received
Unituxin in Study 2.
Neurotoxicity
-
Pain: 114 (85%) patients treated in the Unituxin/RA group experienced pain despite pre-treatment with
analgesics including morphine sulfate infusion. Severe (Grade 3) pain occurred in 68 (51%)
patients in the Unituxin/RA group compared to 5 (5%) patients in the RA group. For severe pain,
decrease the Unituxin infusion rate to 0.875 mg/m2/hour.
Discontinue Unituxin if pain is not adequately controlled despite infusion rate reduction
and institution of maximum supportive measures.
-
Peripheral Neuropathy: Severe (Grade 3) peripheral sensory neuropathy occurred in 2 (1%) patients and severe peripheral
motor neuropathy occurred in 2 (1%) patients in the Unituxin/RA group. Permanently discontinue
Unituxin in patients with peripheral motor neuropathy of Grade 2 or greater severity, Grade 3
sensory neuropathy that interferes with daily activities for more than 2 weeks, or Grade 4
sensory neuropathy.
-
Neurological Disorders of the Eye:
-
Neurological disorders of the eye experienced by two or more patients treated with
Unituxin included blurred vision, photophobia, mydriasis, fixed or unequal pupils, optic
nerve disorder, eyelid ptosis, and papilledema. In Study 1, 3 (2%) patients in the
Unituxin/RA group experienced blurred vision, compared to no patients in the RA group.
Diplopia, mydriasis, and unequal pupillary size occurred in 1 patient each in the
Unituxin/RA group, compared to no patients in the RA group. The duration of eye
disorders occurring in Study 1 was not documented. In Study 3, eye disorders occurred in
16 (15%) patients, and in 3 (3%) patients resolution of the eye disorder was not
documented. Among the cases with documented resolution, the median duration of eye
disorders was 4 days (range: 0, 221 days).
-
Interrupt Unituxin in patients experiencing dilated pupil with sluggish light reflex or
other visual disturbances that do not cause visual loss.
-
Upon resolution and if continued treatment with Unituxin is warranted, decrease the
Unituxin dose by 50%.
-
Permanently discontinue Unituxin in patients who experience loss of vision and in
patients with recurrent eye disorder following dose reduction.
-
Prolonged Urinary Retention: Urinary retention that persists for weeks to months following discontinuation of opioids has
occurred in patients treated with Unituxin. Permanently discontinue Unituxin in patients with
prolonged urinary retention that does not resolve with discontinuation of opioids.
-
Transverse Myelitis: Transverse myelitis has occurred in patients treated with Unituxin. Promptly evaluate any
patient with signs or symptoms such as weakness, paresthesia, sensory loss, or incontinence.
Permanently discontinue Unituxin in patients who develop transverse myelitis.
-
Reversible Posterior Leukoencephalopathy Syndrome (RPLS): RPLS has occurred in patients treated with Unituxin.
Institute appropriate medical treatment and
permanently discontinue Unituxin in patients with signs and symptoms of RPLS (e.g., severe
headache, hypertension, visual changes, lethargy, or seizures).
Capillary Leak Syndrome
-
Severe (Grade 3 to 5) capillary leak syndrome occurred in 31 (23%) patients in the Unituxin/RA
group and in no patients treated with RA alone.
-
Depending on severity, manage by immediate interruption, infusion rate reduction or permanent
discontinuation of Unituxin and institute supportive management in patients with symptomatic or
severe capillary leak syndrome.
Hypotension
-
Severe (Grade 3 or 4) hypotension occurred in 22 (16%) patients in the Unituxin/RA group
compared to no patients in the RA group.
- Prior to each Unituxin infusion, administer required intravenous hydration.
- Closely monitor blood pressure during Unituxin treatment.
-
Depending on severity, manage by immediate interruption, infusion rate reduction or permanent
discontinuation of Unituxin and institute supportive management in patients with symptomatic
hypotension.
Infection
-
Severe (Grade 3 or 4) bacteremia requiring intravenous antibiotics or other urgent intervention
occurred in 17 (13%) patients in the Unituxin/RA group compared to 5 (5%) patients treated with
RA alone. Sepsis occurred in 24 (18%) of patients in the Unituxin/RA group and in 10 (9%)
patients in the RA group.
-
Monitor patients closely for signs and symptoms of systemic infection and temporarily
discontinue Unituxin in patients who develop systemic infection until resolution of the
infection.
Bone Marrow Suppression
-
Severe (Grade 3 or 4) thrombocytopenia (39% vs. 25%), anemia (34% vs. 16%), neutropenia (34% vs.
13%), and febrile neutropenia (4% vs. 0 patients) occurred more commonly in patients in the
Unituxin/RA group compared to patients treated with RA alone.
- Monitor peripheral blood counts closely during Unituxin therapy.
Electrolyte Abnormalities
-
Electrolyte abnormalities occurring in at least 25% of patients who received Unituxin/RA in
Study 1 included hyponatremia, hypokalemia, and hypocalcemia. Severe (Grade 3 or 4) hypokalemia
and hyponatremia occurred in 37% and 23% of patients in the Unituxin/RA group, respectively,
compared to 2% and 4% of patients in the RA group.
- Monitor serum electrolytes daily during therapy with Unituxin.
Atypical Hemolytic Uremic Syndrome
-
Hemolytic uremic syndrome in the absence of documented infection and resulting in renal
insufficiency, electrolyte abnormalities, anemia, and hypertension occurred in two patients
following receipt of the first cycle of Unituxin.
- Permanently discontinue Unituxin and institute supportive management.
Embryo-Fetal Toxicity
- Unituxin may cause fetal harm.
- Advise pregnant women of the potential risk to a fetus.
-
Advise females of reproductive potential to use effective contraception during treatment, and
for two months after the last dose of Unituxin.
ADVERSE REACTIONS
The most common serious adverse reactions (≥ 5%) are infections, infusion reactions, hypokalemia,
hypotension, pain, fever, and capillary leak syndrome.
The most common adverse drug reactions (≥ 25%) in Unituxin/RA compared with RA alone are pain
(85% vs. 16%), pyrexia (72% vs. 27%), thrombocytopenia (66% vs. 43%), lymphopenia (62% vs. 36%),
infusion reactions (60% vs. 9%), hypotension (60% vs. 3%), hyponatremia (58% vs. 12%), increased
alanine aminotransferase (56% vs. 31%), anemia (51% vs. 22%), vomiting (46% vs. 19%), diarrhea (43%
vs. 15%), hypokalemia (43% vs. 4%), capillary leak syndrome (40% vs. 1%), neutropenia (39% vs. 16%),
urticaria (37% vs. 3%), hypoalbuminemia (33% vs. 3%), increased aspartate aminotransferase (28% vs.
7%), and hypocalcemia (27% vs. 0%). In post-approval use of Unituxin, the adverse reactions of
prolonged urinary retention, transverse myelitis, and reversible posterior leukoencephalopathy
syndrome were observed. Because these reactions are reported voluntarily from a population of
uncertain size, it is not always possible to reliably estimate their frequency.
UTXISIhcpJAN18
Please see
Full Prescribing Information
, including Boxed WARNING, for Unituxin.